Mascular Dystrophy
Stem cell based therapies to treat muscular dystrophy
Muscular dystrophies comprise a heterogeneous group of neuromuscular disorders, characterized by progressive muscle wasting, for which no satisfactory treatment exists. Multiple stem cell populations, both of adult or embryonic origin, display myogenic potential and have been assayed for their ability to correct the dystrophic phenotype. To date, many of these described methods have failed, underlying the need to identify the mechanisms controlling myogenic potential, homing of donor populations to the musculature, and avoidance of the immune response. Recent results focus on the fresh isolation of satellite cells and the use of multiple growth factors to promote mesangioblast migration, both of which promote muscle regeneration. Throughout this chapter, various stem cell based therapies will be introduced and evaluated based on their potential to treat muscular dystrophy in an effective and efficient manner.
Introduction
Numerous types of muscular dystrophy exist and differ depending on their degree of severity and the muscle types affected. Duchenne muscular dystrophy (DMD), the most common form of muscular dystrophy, is an X-linked genetic disorder that occurs at a rate of approximately 1 in 3500 male births. DMD arises due to either spontaneous mutations or inherited nonsense point mutations in the dystrophin gene, the result of which is progressive muscle wasting and weakness attributed to the loss of a functional dystrophin protein. Dystrophin, an important cytoskeletal protein, and a major component of the dystrophin–glycoprotein complex (DGC), is responsible for the maintenance of cell integrity, mediation of cytoplasmic signaling and muscle cell function. Without dystrophin, muscle cells cannot form the DGC and degenerate as a result of mechanical stress during contraction.
To test prospective therapeutic treatments for DMD numerous large and small animal models have been created; the most common being the mdx mouse, which parallels DMD defects seen in diaphragm muscle as a result of a genetic mutation causing premature termination of the dystrophin transcript. Although the mdx mouse lacks a functional dystrophin protein, it only displays a mild dystrophic phenotype, which is attributed to a greater degree of fiber regeneration and a reduction in endomysial fibrosis compared to DMD. More recent mouse models include the utrophin/dystrophin null mouse, and the dystrophin/α7-integrin double mutant mouse, both of which more closely resemble human DMD. Feline, zebrafish, and the canine X-linked model of muscular dystrophy, complement the mouse models and provide researchers with additional tools to study this disease.
The function of stem cells in development and tissue homeostasis
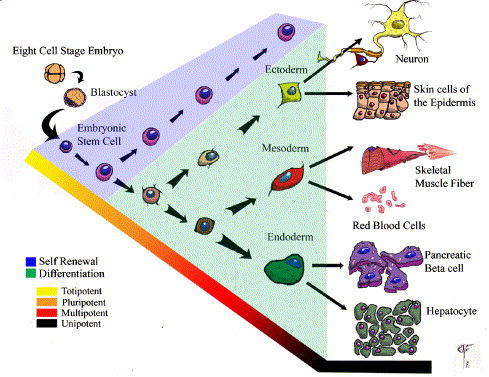
Stem cells are defined by certain characteristics, primarily an ability for long term self renewal and the capacity to differentiate into multiple cell lineages. Stem cells are responsible for the development and maintenance of tissues and organs and self-renew or differentiate in response to a combination of biochemical signals and biomechanical stimuli provided by the stem cell niche. Stem cells can be isolated from either adult or embryonic tissue, and depending on a hierarchical state differ in their ability to give rise to multiple cell lineages. This hierarchy progresses from a state of totipotency through to unipotency; whereby, at each level the ability to differentiate into multiple cell types is progressively diminished (Fig. 1). Stem cell division can be either symmetric or asymmetric. An asymmetric division results in the formation of two non-identical daughter cells; one commits to a specialized fate while the other remains quiescent to maintain the stem cell pool. Conversely, differentiating daughter cells undergo symmetric divisions giving rise to a reservoir of precursor cells that contribute to tissue regeneration.
Small quantities of adult stem cells exist in most tissues throughout the body where they remain quiescent for long periods of time prior to being activated in response to disease or tissue injury. Adult stem cells can be isolated from cells of the hematopoietic, neural, dermal, muscle, and hepatic systems. It is traditionally thought that adult stem cells give rise to the specialized cell types of the tissue from which they originated. However, some recent reports have indicated that adult stem cells can differentiate into lineages other then their tissue of origin, for example transplanted bone marrow or enriched hematopoietic stem cells (HSCs) are reported to give rise to cells of the mesoderm, endoderm and ectoderm. Future experiments elaborating upon the origins and characteristics of adult stem cells are necessary in order to fully distinguish their potential from embryonic stem cells.
The embryonic stem cell (ESC) is defined by its origin—the inner cell mass of the blastocyst. ESCs traditionally differ from adult stem cells in that they are deemed pluripotent; meaning they can give rise to cells derived from all three germ layers. Gene expression patterns observed during the in vitro differentiation of ESCs mimic that seen in vivo; and these cells can give rise to numerous cell types in vitro including neurons, bone, pancreatic islets, and skeletal muscle.
In the past multiple stem cell populations have been assayed for their ability to treat muscular dystrophy, the majority of which have met with limited success. In order to correct the dystrophic phenotype, transplanted cells must fuse to existing, or form new, myotubes. Upon fusion, the contribution of genetically normal myonuclei to the muscle myofiber should result in the production of a functional dystrophin protein. Stem cell based therapies for the treatment of muscular dystrophy can progress via two strategies. The first involves cells from a patient afflicted with DMD and is termed autologous stem cell transfer. In this process cells from the patient are genetically altered in vitro to restore dystrophin expression and subsequently re-implanted. In the second strategy, allogenic stem cell transfer, cells are isolated from an individual with functional dystrophin and subsequently transplanted into a dystrophic patient. Both of these strategies have advantages and disadvantages. Autologous cells are advantageous in that they are derived from the patient and therefore unlikely to elicit an immune response. However, the process of genetic alteration has in the past led to undesirable effects including transformation of donor cells and even death. Allogenic cells, on the other hand, are not subject to genetic modification, making them ideal for functional muscle regeneration. However, the patient is at risk for immune rejection, raising the issues of donor compatibility and appropriate immunosuppressive regimes. In this chapter, multiple sources of stem cells with myogenic potential will be identified and their candidacy as cell sources to treat muscular dystrophy will be assessed. The identification of a stem cell population that provides efficient and effective muscle regeneration is critical for the progression of stem cell based therapies to treat muscular dystrophy.
Skeletal muscle regeneration
Adult skeletal muscle is capable of a remarkable degree of regeneration, suggesting the presence of a stem cell population either resident within muscle or capable of migrating to muscle. The major component of adult skeletal muscle is the myofiber; a giant syncytial cell containing hundreds of myonuclei within a continuous cytoplasm. Under physiological conditions the ability of adult muscle to undergo regeneration is largely attributed to a distinct subpopulation of myogenic cells, termed satellite cells, located between the basal lamina and sarcolemma of mature skeletal muscle fibers. Despite the fact that satellite cells are multipotent in that they can give rise to osteogenic, chondrogenic and adipogenic cells under appropriate conditions, they are a distinct lineage of myogenic stem cells that remain mitotically quiescent under normal physiological conditions. Upon muscle damage or in a state of disease, satellite cells activate and proliferate giving rise to a population of cells that contribute to muscle regeneration via a process of differentiation and fusion. Satellite cells can be characterized by a panel of cell surface markers including: M-cadherin, c-Met, Syndecan 3 and 4, CD-34, and nuclear markers Pax7, MNF, and Myf5. Patients afflicted with DMD rapidly exhaust their satellite cell reserves due to continuous cycles of muscle injury and regeneration. and as such lose their ability to regenerate, resulting in compromised muscle function and degeneration.
Applications of typical muscle stem cells
Transplantation of satellite cell derived myoblasts
Satellite cells are present at low quantities in adult muscle and account for 2–5% of sublaminar nuclei associated with myofibers. Due to their scarcity and the difficulties in isolating pure populations, freshly isolated satellite cells have been largely neglected as a source for cell therapy. The progeny of muscle satellite cells, upon culture and expansion in vitro, are termed primary myoblasts, these cells are highly proliferative and can be maintained in an undifferentiated state. Historically primary myoblasts have been the principal source of muscle progenitors for cell-based therapies aimed at treating muscular dystrophy. Myoblast transplantation (MT) involves the delivery of primary unmodified skeletal myoblasts to muscle typically via an intramuscular injection. This method is advantageous in that muscle biopsies are easily conducted on limb musculature, techniques for genetic modification of myoblasts are efficient, and large quantities of in vitro expanded myoblasts are easily achieved.
The potential of MT originates from initial experiments performed in mice which demonstrated the capacity of donor myogenic cells to regenerate recipient muscle. Experiments conducted by Partridge et al. using the immortal C2C12 mouse myoblast cell line validated the ability of exogenous myoblasts to induce synthesis of dystrophin in dystrophin-deficient mdx muscle fibers. Subsequent experiments confirmed and elaborated upon these results by using myoblasts from newborn or adult mice in addition to human myoblasts as donor cells for transplant into mdx mice. These experiments demonstrated the ability to track the transplanted cells in the host through the use of LacZ staining, or in the case of human myoblast transfer antibodies specific for human dystrophin. MT was later tried in non-human primates in order to assess the regenerative capacity and immune response involved. Primate derived myoblasts successfully integrated into allogenic hosts when injected 1 mm apart and in combination with the immunosuppressive FK506. These experiments indicate that primate derived myoblasts could integrate into regenerating muscle and survive after 1 year, however none of these experiments provide any evidence as to whether the transplanted cells provide any physiological correction of the dystrophic phenotype.
On the basis of research conducted in mice and nonhuman primates, human clinical trials involving the transplantation of myoblasts were initiated in the early 1990s. Initial trials involved repetitive intramuscular injections of large quantities of myoblasts (> 106 cells) distributed over multiple sites. Although reported as successful, functional evidence was elusive and plagued with false positives resulting from revertant fibers, which arise from a second mutation and occur due to either a somatic deletion or through splicing of further exons in the dystrophin gene. These events lead to the restoration of the reading frame allowing for the production of a truncated, yet partially functional dystrophin molecule. Later clinical trials involved techniques to distinguish dystrophin-positive fibers derived from donor DNA from host revertant fibers. These techniques eliminate confusion concerning the contribution of donor cells to muscle regeneration and allow for a more confident assessment of physiological benefit post transplantation.
The majority of past experiments involving myoblast transfer to treat DMD failed to show substantial physiological correction of the dystrophic phenotype. Although, recent clinical attempts show improvement in the areas of cell survival, migration, and evasion of the immune response, these issues remain at the forefront of myoblast transplantation. Since grafted myoblasts have limited migration, repeated local injections are required to treat a significant portion of the myofibers in any given muscle. Considering DMD patients succumb to heart and diaphragm failure, repeated injections 1 to 2 mm apart would be required in these muscles to ensure patient survival, a technique that is currently beyond our grasp. In addition, transplanted myoblasts do not participate in long term muscle regeneration making them less than ideal for the treatment of DMD. In conclusion, while myoblast transfer provides transient delivery of dystrophin and improves the strength of injected dystrophic muscle it is considered an interim solution to ease the suffering of patients with muscular dystrophy. In order to be considered a viable widespread treatment option for DMD, myoblasts must contribute to multiple rounds of regeneration and be conducive to widespread distribution throughout the musculature.
Satellite cell transplantation
The ability to directly isolate a pure population of satellite cells from diaphragm muscle, by using a Pax3-GFP knock-in mouse [88], was recently accomplished. This Pax3-GFP mouse incorporates the green fluorescent protein (GFP) under the control of the Pax3 promoter allowing faithful recapitulation of Pax3 expression. The use of fluorescent activated cell sorting (FACS) permits the purification of a GFP positive population of Pax3+/CD34+/Pax7+ cells. Based on gene expression, these results suggest the isolation of a predominantly quiescent population of satellite cells. When injected into dystrophic muscle, this population of cells is capable of restoring dystrophin expression 3 weeks post-transplantation. Importantly, the yield of dystrophin expressing muscle obtained when small numbers of isolated satellite cells were transplanted into irradiated muscle was significant. Freshly isolated satellite cells not only restored dystrophin expression in mdx mice but also formed roughly 17% of the satellite cell pool expressing both Pax7 and Pax3-GFP; an indication that donor cells were capable of contributing to the muscle satellite cell compartment. Moreover, approximately 25 fold more cells are needed to obtain similar levels of regeneration from donor cells isolated by enzymatic dissociation of whole adult muscles, as opposed to grafting Pax3-GFP sorted cells.
However, the full potential of this approach is affected by several limitations. First, the cultivation of freshly isolated satellite cells in vitro significantly reduces their in vivo myogenic potential; therefore, whether or not sufficient numbers of donor satellite cells can be obtained is a key issue. The isolation of sufficient quantities of Pax3-GFP satellite cells is difficult because these cells can only be isolated from the diaphragm and body trunk muscles but not from limb muscles. In fact, the current absence of appropriate cell surface markers to identify a Pax3+/CD34+/Pax7+ population of satellite cells makes this isolation technique impossible in humans. Given that genetic manipulations generally require short-term cultivation in vitro, and in vitro culture decreases the regenerative potential of Pax3-GFP populations, then genetic correction of autologous sorted satellite cells does not appear to be a viable option. This is particularly important from a clinical standpoint since cell transplantation of autologous genetically corrected satellite cells to DMD patients is theoretically the ideal approach to minimize host immune rejection of donor cells. A clinically relevant approach to using fresh satellite cells would involve their isolation from the peripheral musculature, based on a panel of cell surface markers, subsequent culture in vitro under conditions that promote the maintenance of their stem cell state, followed by gene therapy prior to transplantation. In the absence of cell surface markers to isolate quiescent satellite cells from the musculature this alternative is currently not an option; therefore, research into the identification of a feasible isolation strategy is of the utmost importance.
Single muscle fiber
Experiments conducted in the early 1980s involving the transplant of whole muscle indicated that resident satellite cells are capable of initiating regeneratio. While, enzymatic dissociation and the subsequent transplantation of satellite cells from myofibers results in marginal muscle regeneration, the transplantation of satellite cells still associated with a single muscle fiber (containing as few as seven satellite cells) can generate in the range of 100 myofibers with thousands of corresponding myonuclei. Interestingly, the satellite cells resident upon a transplanted myofiber will contribute to the host satellite cell compartment and be available for multiple rounds of regeneration. Transplanted satellite cells appear to migrate throughout the muscle in which the myofibers were implanted; however, no direct quantification of the migratory potential of donor satellite cells exists. The notion that single muscle fibers will be used to treat muscular dystrophy does not in itself present a realistic therapeutic approach. Questions regarding the procurement of donor muscle fibers is somewhat belied by the large regenerative potential of individual satellite cells. However, no evidence suggests donor satellite cells are able to populate neighboring muscles; indicating that this method of cell transplant would involve multiple transplantations.
In general the data presented in this section provides evidence that quiescent satellite cells maintained in their niche retain a large degree of regenerative potential. Once it is possible to simulate the satellite cell niche in vitro the real potential of satellite cells could be harnessed for therapeutic purposes. Experiments conducted with fresh satellite cells as well as intact muscle fibers allude to the necessity of identifying the molecular mechanisms responsible for satellite cell self renewal and differentiation. The drastic increase in regenerative potential from either freshly isolated satellite cells or intact myofibers suggests a link between the maintenance of the satellite cell niche and the efficiency of muscle regeneration. Future experiments to identify the components of the satellite cell niche that are responsible for the activation or maintenance of satellite cells in a quiescent state will be of great importance for the validation of satellite cell based therapies.
Applications of atypical muscle stem cells
Muscle side population cells
Within muscle, in addition to satellite cells, there exists a population of stem cells that possess myogenic potential, termed side population (SP) cells. This stem cell population, isolated by FACS based on its exclusion of the Hoechst 33,342 dye (via the ABC transporter Bcrp1/ABCG2), can be isolated from many adult tissues. SP cells isolated from bone marrow (bmSP) or muscle (mSP) on their own are unable to undergo myogenic differentiation in vitro, yet upon intramuscular transplantation can give rise to both myocytes and satellite cells. The mSP population when isolated from a Pax7−/− background, where satellite cells are absent, and co-cultured with myoblasts or forced to express MyoD undergo muscle specification. These data suggest mSP cells and satellite cells constitute distinct populations that progress along different myogenic pathways. Studies directly comparing muscle regeneration after intravenous injection of bone marrow side population bmSP and mSP cells indicated a reduced ability of the mSP fraction to reconstitute the hematopoietic compartment in lethally irradiated mice; however, both populations regenerate muscle to a similar degree.
In contrast to satellite cells or primary myoblasts, mSP cells are able to migrate from the blood stream into muscle, a desirable feature for widespread distribution of a therapeutic cell type. Intravenous transplantation of mSP cells typically yields at most a 1% engraftment rate, however upon delivery into noninjured, nonirradiated mdx mice via femoral artery catheterization mSP cells engraftment into muscle at rates approaching 5–8% in select muscles. These results provide evidence that, under physiological conditions, the mSP population can provide dystrophin to diseased muscle via arterial transplantation.
One aspect of mSP transplantation is puzzling, if mSP cells can take up the satellite cell position, and this is reported in numerous articles, why do they not appear to contribute to long term muscle regeneration? Perhaps mSP give rise to a committed myogenic satellite cell expressing Pax7 rather then a satellite cell with stem cell-like properties? Further advances in the field of mSP transplantation must address the following issues: low levels of integration following arterial or intramuscular transplantation, an inability to partake in long term regeneration, and achieving physiological improvements to dystrophic muscle. Prior to these issues being resolved mSP cells currently do not constitute a viable cell source to treat DMD.
Bone marrow cells
Bone marrow transplantation (BMT) or hematopoietic stem cell transplantation (HSCT) involves the transplantation of hematopietic stem cell (HSC) in order to produce new blood cells and repopulate the bone marrow. Evidence of a population of circulating cells with myogenic potential present in the bone marrow was identified in the late 1960s. Ferrari et al. (1998) later confirmed that BM-derived cells can, at very low levels, undergo myogenic differentiation and participate in muscle repair after injury. This research presented the idea of delivering donor cells via the circulation to take part in skeletal muscle regeneration; a potentially powerful development considering the daunting task of injecting donor cells into individual muscle masses. The following year studies involving the transplantation of BM-derived cells conducted in the mdx mouse partially restored dystrophin expression. Bone marrow derived cells persist in the musculature for long periods of time and maintain their dystrophin expression, however quantitatively the amount of muscle generated after a BM transplant does not comprise a therapeutically relevant amount when only 0.5% of regenerating fibers contain donor cells.
A clear mechanism detailing the process by which cells in the bone marrow contribute to muscle regeneration remains elusive. Experiments conducted by LaBarge et al. attempted to elaborate on the process by which bone marrow cells contribute to muscle regeneration by the transplantation of bone marrow derived cells (BMDCs) into irradiated SCID mice. BMDCs appear to contribute following muscle irradiation to the satellite cell niche and further exercise induced damage led to the incorporation of BMDCs into multinucleated myofibers at a frequency approaching 3.5%. These initial experiments have been elaborated upon using exercise as opposed to muscle irradiation leading to the conclusion that BMDC can incorporate into muscle under physiological conditions. However, the question remains whether integration of cells from the bone marrow into muscle is a physiologically relevant process. Experiments by Camargo et al. and Corbel et al. (2003) analyzed the ability of bone marrow derived HSCs to participate in muscle regeneration; and while both studies found HSC progeny could incorporate into muscle, this ability is more likely attributed to fusion rather then the existence of a myogenic HSC [101], [102]. Other hematopoietic stem cell populations exist including the CD45+/Sca-1+ population, which following muscle injury undergoes a 30 fold expansion in regenerating muscle and readily undergoes myogenic differentiation in vitro. These experiments further concluded that Wnt signaling molecules play a role in augmenting the myogenic specification of CD45+/Sca1+ cells. Although, later experiments would lead to the conclusion that under physiological conditions bone marrow, and the HSCs contained within, play a minor role in muscle regeneration [104] this technique in combination with appropriate growth factors and suitable methods for transplantation may eventually serve as a method to treat DMD.
Although the major stem cell component of bone marrow is that of the HSC, and the contribution of bone marrow derived cells to the physiological process of muscle regeneration is considered by some to be trivial, the contribution of cells in the bone marrow may differ significantly between mice and humans. AC133, a human cell surface marker for the hematopoietic/endothelial lineages was recently used to isolate a population of cells from human blood that can, upon in vitro co-culture with myogenic cells or exposure to Wnt-producing cells, undergo a degree of myogenic conversion. This finding has reopened the debate on a blood born population with myogenic potential. These AC133+ cells, when co-cultured or exposed to Wnts, display an mRNA pattern reminiscent of that found in satellite cells including: M-cadherin, Pax7, CD34 and Myf5. Whether AC133+ cells constitute a true myogenic progenitor is difficult to determine considering these cells do not undergo myogenic differentiation spontaneously when myogenesis was induced with low serum levels. Nevertheless, limited myogenic conversion and dystrophin expression is observed upon intra-arterial injection or intramuscular injection of AC133+ cells. This method offers certain advantages over other HSC populations including: engraftment of donor cells under physiological conditions exceeds that shown previously for bone marrow, HSCs or mSP cells, and functional tests of injected muscles revealed a substantial recovery of force after treatment. Considering these qualities this method to treat DMD warrants further analysis regarding the process by which the AC133+ population contributes to myogenic regeneration, the localization of AC133+ cells within the circulatory system, and the ability to expand ex vivo these cells prior to transplantation.
Mesenchymal stem cells
Multipotent mesenchymal stem cells (MSCs), first derived from bone-forming progenitor cells resident in the bone marrow, are capable of producing skeletal muscle in addition to osteoblasts, chondroblasts, and adipocytes [106], [107], [108]. Typical methods for MSC isolation involve percoll fractionation and subsequent culture with varying growth factors [109]. The debate surrounding the pluripotent nature of MSCs continues; indeed reports suggest a population of cells co-purified with MSCs can, in vitro, differentiate into visceral mesoderm, neuroectoderm and endoderm [110]. These pluripotent stem cells are termed multipotent adult progenitor cells or MAPCs. MAPCs appear to reside in the brain, skeletal muscle, and bone marrow of human and mouse tissues [111]. Being adult derived; MAPCs avoid many ethical and immunological hurdles associated with embryonic stem cells facilitating their therapeutic application. Continuing research must focus on the origins of MAPCs and the molecular mechanisms that govern their development as well as the ability to induce myogenesis from these cells prior to them being a potential therapeutic source to treat DMD.
Other MSC populations exist and can be isolated via enzymatic digestion and serial passaging of cells from adult human synovial membrane (hSM-MSCs) [112]. These hSM-MSCs possess multilineage potential in vitro and recapitulate the temporal gene expression typical of embryonic myogenesis when directly injected into injured TA muscles of immunosuppressed mdx mice [113]. hSM-MSCs can engraft into regenerating muscle, express dystrophin, and give rise to putative satellite cells that persist for 6 months after transplantation. However, the gene expression profile of hSM-MSC derived satellite cells must be clarified considering neither M-cadherin, Pax7, or CD34 are shown to be expressed in these cells. A method to resolve this issue would be to conduct single cell clonal analysis to confirm the ability of hSM-MSC derived satellite cells to proliferate and give rise to multi-nucleate myotubes. At this point in time additional research into the physiological benefits of hSM-MSCs post transplantation, as well as the confirmation of satellite cell characteristics are necessary in order to progress therapeutically with this technique.
In addition to the MSC types stated above there exists a method to convert MSCs to the skeletal muscle lineage [114] via infection with activated Notch in the presence of various cytokines. Notch is a transmembrane protein, which upon binding with its ligands Delta or Jagged, undergoes cleavage to release an intracellular domain (NICD) in order to effect downstream signaling. The Notch signaling pathway is involved in embryonic tissue morphogenesis [115], adult cell fate selection [116], and has been linked to satellite cell activation and muscle differentiation, making it a candidate molecule for the myogenic induction of MSCs [117]. Experiments conducted by Dezawa et al. (2005) demonstrate the ability to generate skeletal muscle progenitors from human and rat MSCs. Single cell clonal analysis confirms the ability of isolated muscle-MSCs (M-MSCs) to form multi-nucleate myotubes following treatment with the NICD and culture in low concentrations of horse serum to induce fusion. Intravenous injection of M-MSCs in immunosuppressed rats, pretreated with cardiotoxin in the gastrocnemius muscle, resulted in their incorporation into newly formed myofibers, and not bone, heart, liver, kidney or undamaged muscle. M-MSCs localize to the sublaminar portion of myofibers, express Pax7 and c-Met via immunofluorescence of separate cellular fields and upon multiple rounds of regeneration contribute to muscle repair.
Questions remain regarding the contribution of M-MSCs under physiological conditions to the functional amelioration of the dystrophic phenotype. Interestingly, M-MSCs express myogenin (a terminal marker of muscle differentiation) at high levels along with MyoD, early markers of muscle formation Six1, and Six4 along with Pax7 a satellite cell marker. Considering their gene expression profile M-MSCs appear to be a heterogeneous population of undifferentiated and partially differentiated myogenic progenitors. Further characterization of M-MSCs and the in vivo derived satellite cells is required to answer these questions. In addition, the use of Notch to induce myogenic specification is a unique approach more commonly associated with the maintenance of quiescent satellite cells or the prevention of terminal muscle differentiation. This is not to say the use of Notch to promote myogenic specification is improper, however without a clear molecular mechanism governing the role of Notch in the myogenic commitment of MSCs nor a basis for converting MSCs to the myogenic lineage without over-expressing the NICD this current route is not practical for the treatment of patients with DMD. This method offers the ability to derive myogenic cells that can be easily obtained and expanded from patients, genetically modified in vitro, and re-introduced via the circulation all beneficial therapeutic characteristics.
In summary, multiple different types of MSCs can be isolated and serve as potential cell types for therapy, however in the absence of a defined group of cell surface markers a reproducible system to isolate cells with myogenic potential from MSCs becomes difficult. The reproducible isolation of pure MSC populations and their downstream differentiation into the muscle lineage holds tremendous potential for the treatment of neuromuscular disorders. Given the rapid progress in the areas of cell purification and characterization therapeutic relevancy may not be far off.
Muscle Derived Stem Cells (MDSCs)
Muscle derived stem cells (MDSCs) are a distinct population of stem cells resident in adult skeletal muscle. MDSCs are thought to reside upstream of satellite cells in the terms of their potency, and are not restricted to either the myogenic or mesenchymal lineages [118], [119]. Numerous muscle derived stem cell populations have been shown to contain hematopoietic potential [93], [120], [121] the most prominent being that derived via the preplate technique [22]. These MDSCs represent a heterogeneous population of cells in terms of their high expression of either Sca1 or CD34. While the physiological location of MDSCs remains unknown they often express MNF and the myogenic regulatory factor MyoD and share certain characteristics with a population of hematopoietic stem cells expressing CD34 and Sca1 [122]. When injected into limb muscle or into the circulation of mdx mice MDSCs contribute to muscle regeneration and express dystrophin [123], [124]. In comparison to transplantations conducted with primary myoblasts, MDSCs show a 10 fold increase in dystrophin expression and incorporate into vessels and surrounding nerves [22].
Although certain characteristics of MDSCs including their ease of proliferation in vitro, ability to migrate through the vasculature, and their multipotentiality are amenable to therapeutic applications, a lack of physiological improvement to the dystrophic condition coupled with long term self renewal resulting in their transformation mar the use of MDSCs to treat DMD [125], [126]. In an attempt to determine the physiological location of MDSCs, some reports claim they originate in the bone marrow and reside in the musculature [120] while others claim they are muscle derived [126], [127]. Further research into the origins of MDSCs is required prior to the clinical use of these cells.
Vessel associated stem cell populations
Cells with endothelial and myogenic properties exist and can be isolated at embryonic, fetal [128], and postnatal stages [129] (3 weeks) of development yet the identification of a bona fide adult vessel derived stem cell remains elusive. In 1999 De Angelis et al. discovered a stem cell population resident in the embryonic dorsal aorta having both endothelial and myogenic markers [130]. Although, explant cultures of the dorsal aorta do not initially express any myogenic markers; upon differentiation in culture they gain both endothelial and myogenic markers known to be present on adult muscle satellite cells. Surprisingly, the quantity of satellite cells produced in vitro from the dorsal aorta is in the range of 50 fold more than that derived from somitic explants [130]. Dorsal aorta derived myogenic cells can in vitro fuse with primary myoblasts during differentiation and when transplanted into TA muscles contribute their nuclei to muscle regeneration [130].
Further work on vessel associated stem cells isolated from the dorsal aorta demonstrated their multi-potentiality leading to their classification as mesangioblasts[131]. Studies conducted by Sampolesi et al. using immunocompetent α-sarcoglycan null mice which serve as a model system to study limb-girdle muscular dystrophy indicated that the intra-arterial injection of mesangioblasts results in their migration throughout the vasculature, giving rise to both morphological and functional correction of the dystrophic phenotype, a quality absent in other myogenic stem cell populations [132]. This method was further improved by the treatment of mesangioblasts with either stromal-derived factor (SDF) 1 or tumor necrosis factor (TNF) α which resulted in enhanced transmigration in vitro and migration into dystrophic muscle in vivo [133]. The combination of pretreatment with TNFα and SDF-1 and the expression of α-4 integrin lead to remarkable (∼50%) incorporation of arterially transplanted mesangionblasts into α-sarcoglycan deficient muscle. Long term survival of pre-treated mesangioblasts is observed in muscle masses after 4 months, with α-sarcoglycan mice expressing ∼60% of the α-sarcoglycan detected in a wild type mouse. Although these reports are extremely promising, confirmation of the human equivalent of mesangioblasts that can be isolated from adult tissue is of great importance.
Pre-treated mesangioblast can migrate through the vasculature, engraft at therapeutic levels in muscle, persist and contribute to long term regeneration, and upon genetic modification of autologous cells do not elicit an immune response. Due to these qualities mesangioblasts constitute a potential therapeutic cell type to treat DMD. Prior to their use in clinical trials effective methods to isolate mesangioblasts from adult human tissue must be established, information regarding their long term contribution to muscle function, and the effects of unwanted penetration into non desired cell types must be resolved.
Research supporting the vascular origins of myogenic stem cell populations provides therapeutic potential for the systematic delivery of myogenic progenitors. Whether or not these populations (embryonic, fetal or post natal) of vessel associated stem cells are unique, or derived from an upstream stem cell remains to be determined. Considering these cells are associated with the vasculature it is not a stretch to imagine stem cell populations with myogenic potential residing in multiple adult tissues. While the exact characteristics of vessel associated stem cells remains a mystery their ability to migrate throughout the vasculature coupled with their myogenic potential make them an attractive source for use in cell therapy.
Embryonic stem cells
Small quantities of skeletal muscle were first derived from murine embryonic stem (ES) cells nearly 20 years ago [40], however since then few advances have been made to improve the efficiency of the process. First reports regarding gene expression patterns, functional properties, and morphology of ES derived skeletal muscle parallel observations in vivo. Gene targeting in ES cells is often used to assess gene function, and as a result numerous ES cell lines with modifications to genes important in myogenesis exist. Typically, created to study embryonic development in the mouse these ES cell lines were analyzed during in vitro differentiation to study the molecular mechanisms involved in embryonic myogenesis. Over time limited inhibitors and activators of skeletal muscle formation from ES cells were identified along with strategies to harness their potential for regenerative medicine.
One study conducted by Barberi et al. and published in 2005 shows therapeutic potential and involves the derivation of skeletal muscle by way of hES derived mesenchymal stem cells. This method offers the ability to derive unlimited numbers of pure MSCs from hESCs, and their subsequent differentiation into the skeletal muscle lineage. In order to obtain skeletal muscle from hES derived MSCs (hESMPCs) either co-culture or conditioned medium from the murine myoblast cell line C2C12 or the addition of 5-AzaC as a demethylating agent is required. Currently these experiments do not address the ability of hESMPCs to give rise to Pax7 positive cells nor their ability to contribute to muscle regeneration in vivo.
ES cells to date have not had a significant impact on the development of cell-based therapies to treat muscular dystrophy. Currently one study exists involving differentiated ES cells (3 days) co-cultured with dissociated skeletal muscle fibers for 4 days prior to transplantation into mdx muscle. After 2 weeks dystrophin expression was detected in select regions over and above that which would occur naturally from revertant fibers. These experiments represent the preliminary steps for the use of ES cells to treat DMD and currently do not demonstrate any indication of the long-term regenerative capacity of transplanted cells. Questions remain as to the mechanism by which ES cells, co-cultured in the presence of adult skeletal muscle, contribute to the process of muscle regeneration.
Currently no methods exist to generate large quantities of skeletal muscle from ES cells and for this reason the ES cell model system remains better suited as a tool to study embryonic myogenesis. Potential benefits of using ES cells to treat muscular dystrophy focus on the isolation of large quantities of myogenic stem cells, their ease of genetic modification in vitro, and the ability to derive immune matched cell lineages for transplant. Although the creation of patient specific hES cell lines remains mired in controversy, the principle retains a high degree of feasibility and in the interm progress on the mechanisms involved in myogenic induction and differentiation continue.
Synergistic methods to improve stem cell based therapies
In this chapter we have presented numerous stem cell types with varying degrees of myogenic potential. Irrespective of their source, these stem cell populations share certain hurdles to overcome prior to their therapeutic use. Survival and the subsequent migration from the site of injection remains suboptimal for many of the cell populations outlined previously and from a clinical standpoint the immune response generated upon introduction of a foreign cell type, or in the case of DMD a foreign protein, is a concern. As the mechanisms surrounding the survival and proliferation of myogenic cells post transplant are unraveled, and appropriate growth factors are identified the success of cell based therapies to treat muscular dystrophy will improve. Mechanisms involved in the migration of donor cells to skeletal muscle and the satellite cell niche are still poorly understood. Although some stem cell types, namely mesangioblasts, mSP cells, M-MSCs, have the ability to migrate through the vasculature, most do not. Potential future avenues to increase the migratory ability of stem cell populations include the identification of cell surface markers (e.g., l-selectin+ cells) and appropriate growth factors. As is observed in the case of mesangioblasts, pre-treatment with the growth factors TNFα and SDF-1 led to a substantial improvement in their migratory abilities.
Cell transplantation often elicits an immune response, and in addition to immunosuppressive drugs, there exist methods to overcome immune rejection. One such method involves the establishment of central immune tolerance through a process of mixed hematopoietic chimerism. The mechanism surrounding mixed hematopoietic chimerism is not fully understood yet the procedure is well established in animal models and pursued in the clinic. Matching the genetic background of the various stem cell derived myogenic precursors with hematopoietic stem cells could potentially allow the tolerisation of patients to myogenic transplants.
Irrespective of the stem cell population chosen to treat muscular dystrophy the above-mentioned characteristics (survival, localization, and immunogenicity) remain. In order for the chosen cell type to be successful it must be optimized to deal with these issues. The identification of suitable growth factors, appropriate surface markers, and methods to escape immune detection will be of great importance for the progression of this field.
Conclusions and future perspectives
Stem cell therapy is an attractive method to treat muscular dystrophy because in theory only a small number of cells, together with a stimulatory signal for expansion, are required to elicit a therapeutic effect. In order to achieve clinical relevance candidate stem cell populations must be easily obtained, upon isolation remain capable of efficient myogenic conversion, and when transplanted must integrate into the musculature leading to the functional correction of the dystrophic phenotype
Stem cell populations with myogenic potential can be derived from multiple regions of the body at various stages of development (Fig. 2). Many questions linger regarding the mechanisms by which atypical, or non satellite cell derived precursors, participate in muscle regeneration. A general consensus in the field identifies satellite cells as the primary, if not only, physiologically relevant population that contributes significantly to muscle regeneration. However, because satellite cells are not currently amenable for distribution throughout the vasculature they do not constitute a viable option to treat DMD. A superficial comparison of the atypical myogenic stem cell populations reveals a common theme whereby a cell often linked to the circulatory system is able to migrate to regenerating muscle and contribute in a limited way to this process. The localization of adult mSP, MDSCs, Mesangioblasts, Bone Marrow derived HSCs and HSC populations expressing the cell surface marker AC133 remains unknown. The idea that myogenic potential resides in cells associated with the vasculature is not novel, in fact pericytes which line the capillaries appear to possess qualities of multipotent mesenchymal progenitors If indeed pericytes take part in myogenic regeneration this could explain the widespread distribution of atypical stem cell populations with myogenic potential.
In conclusion, upon comparing the prospective stem cell populations with myogenic potential, the cell type that fulfills the most criteria for use in the treatment of DMD is the mesangioblast. Mesangioblasts serve as a paradigm for widespread distribution, and upon growth factor pre-treatment are able to correct significantly the dystrophic phenotype. Perhaps the application of growth factor pre-treatment to other myogenic stem cell populations may improve their ability to treat muscular dystrophy, however for now mesangioblasts serve as a beacon of hope for patients suffering from various muscular dystrophies.
Our Products




